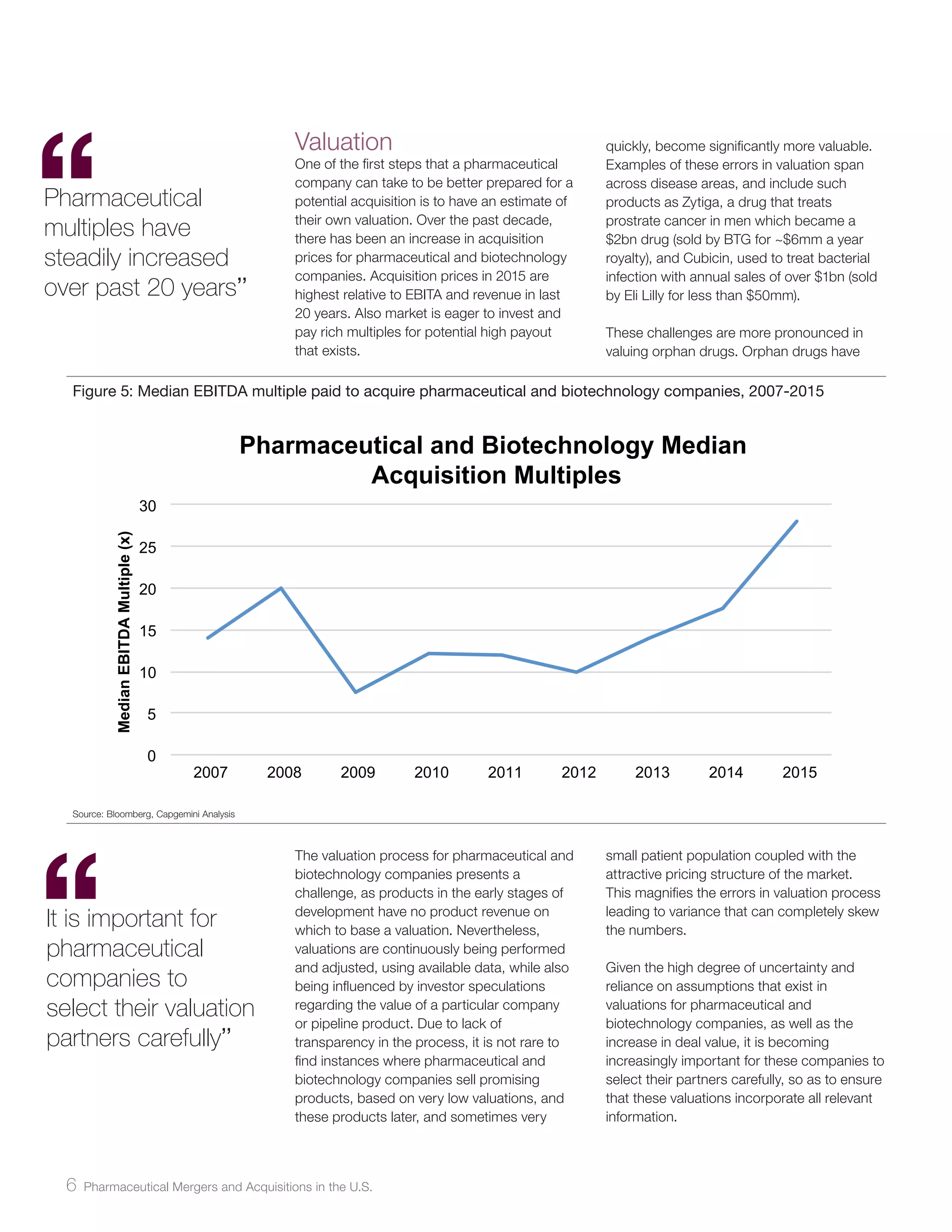
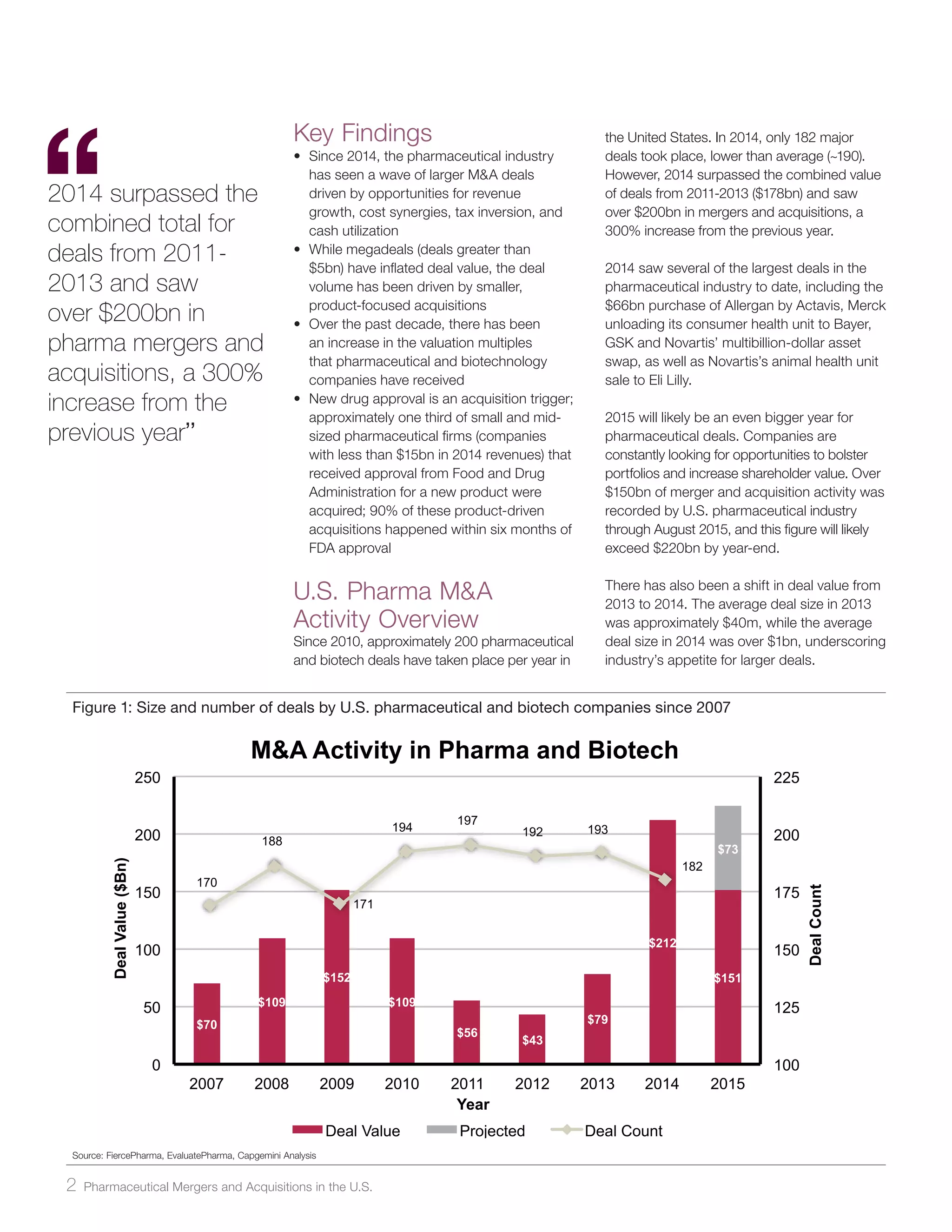
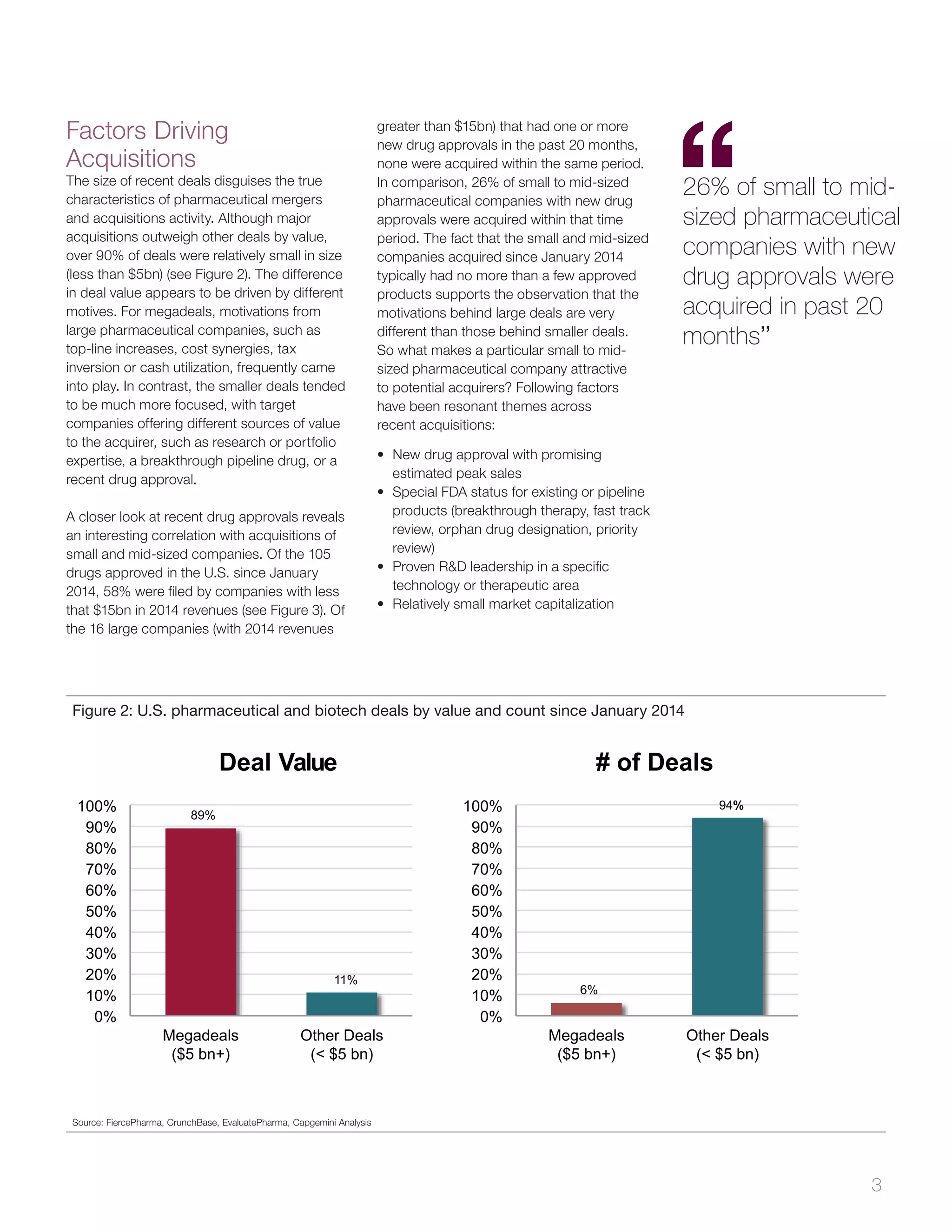
The document analyzes pharmaceutical mergers and acquisitions (M&A) in the U.S., highlighting a significant increase in deal value driven by larger acquisitions since 2014, which contrasts with a predominant number of smaller, product-focused deals. The correlation between new drug approvals and M&A activity is emphasized, noting that one-third of small and mid-sized firms with FDA approvals were acquired shortly after. It suggests that companies need to prepare for potential acquisitions by understanding their market value, the nature of their products, and maintaining strategic relationships.

![4 Pharmaceutical Mergers and Acquisitions in the U.S.
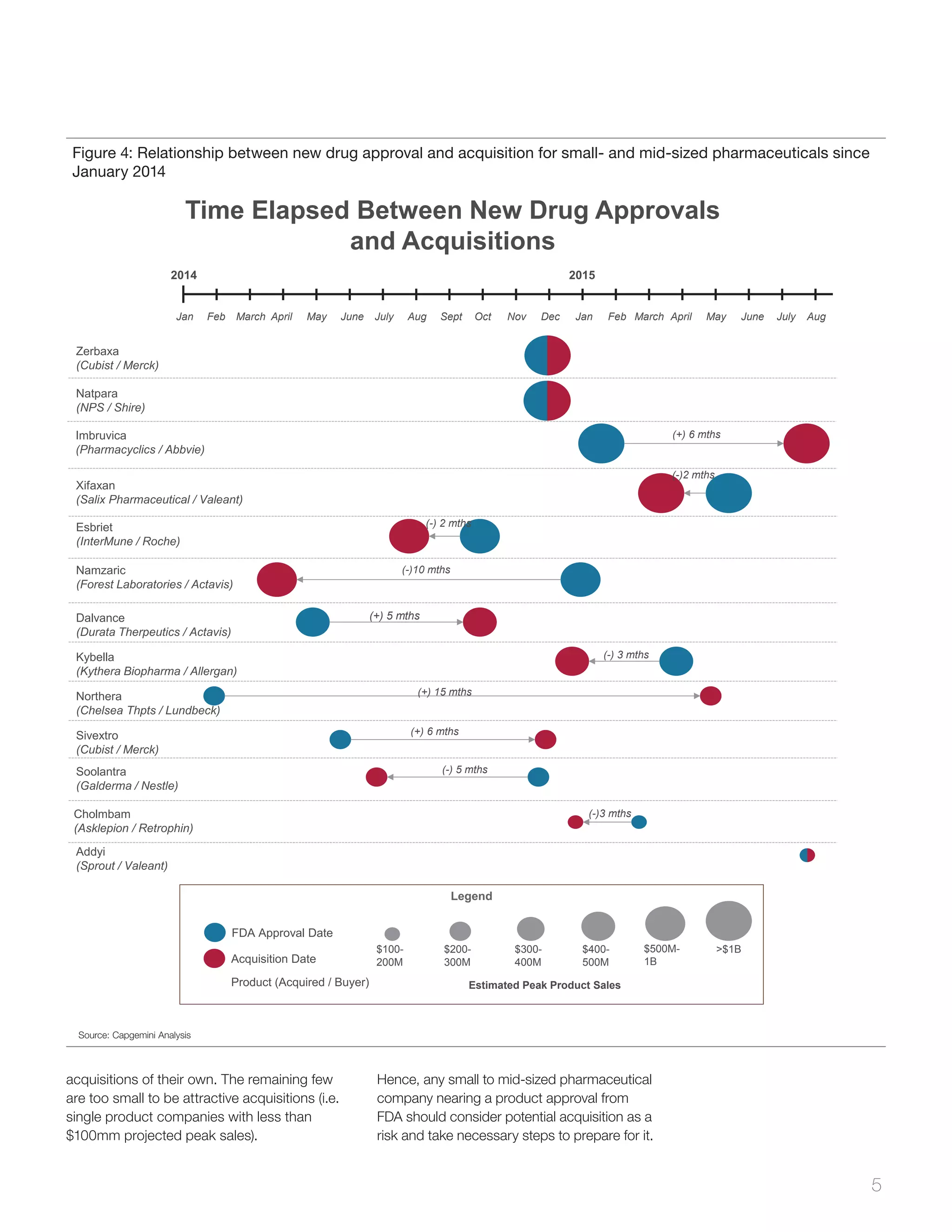
Figure 3: New drug approvals by FDA since January 2014 across companies
Source: FDA, Capgemini Analysis
New Drug Approval as
an Acquisition Trigger
Any acquisition is a result of various
interrelated factors. Failure of bigger
pharmaceutical companies to consistently
develop new drugs and pressure from
shareholders to deliver returns have forced
large pharmaceutical companies to look
outside for innovative drugs. This has resulted
in new drug approvals emerging as a major
trigger for acquisitions. Capgemini Consulting
collected the data for all the new drug
approvals from January 2014 through August
2015 to analyze the correlation between new
drug approvals and acquisitions. Companies
that received the approvals were segmented
into large, mid-size and small based upon
their 2014 revenues (large >=$15bn, $15bn <
mid <$5bn, small <=$5bn).
Since there were no acquisitions in the large
segment over last 20 months, the analysis
focuses on the small to mid-sized
pharmaceutical companies. Figure 4 shows
the correlation between small to mid-sized
pharmaceutical companies, that were
acquired in last 20 months with the new
drug approvals.
As seen in Figure 4, all companies, with the
exception of Chelsea Therapeutics, were
acquired within six-months of obtaining FDA
approval. Excluding Forest Laboratories, in
cases where a company was acquired prior to
drug approval [denoted by (-)], the period
between approval and acquisition was less
than six months, meaning that the drug was
under FDA review when the deal was finalized.
Not surprisingly, in the two cases (Zerbaxa and
Natpara) where the drug was expected to be a
blockbuster, the company was acquired within
days of receiving FDA approval.
Of the small and mid-sized companies with
new drug approvals that have not yet been
acquired, four have already been predicted by
Wall Street as the next probable targets.
Furthermore, some of the companies with
new drug approval possess certain
characteristics that make them unattractive
acquisition targets. Some companies, such
as Mannkind and Pharming Group, have
strategically established relationships with Big
Pharmaceutical companies that deter other
companies from attempting an acquisition.
Others, such as Knight Therapeutics and The
Medicines Company, have opted to increase
their market capitalization through
Most small to mid-
sized pharmaceutical
companies that
received new drug
approval from FDA
were acquired within
six months.’’
42%
13%
45%
Recent Drug Approvals (since January 2014)
Large Pharma (2014
Revenue > = $15bn)
Mid-Sized Pharma
Small Pharma companies
(2014 Revenue < = $5bn)](https://image.slidesharecdn.com/pharmaceuticalmergersacquisitionsintheu-151203082832-lva1-app6892/75/Pharmaceutical-Mergers-Acquisitions-in-the-U-S-4-2048.jpg)