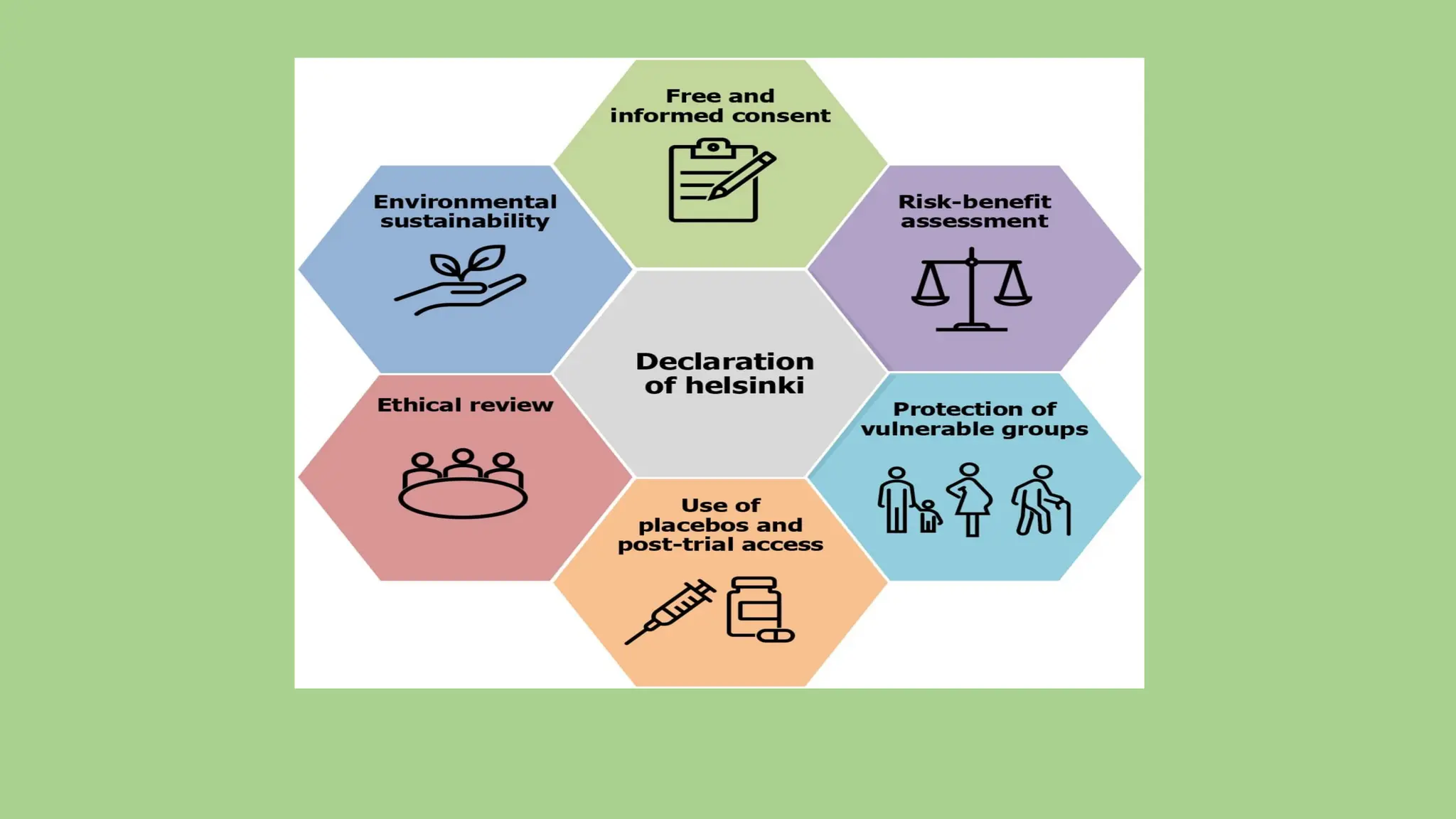
Declaration of Helsinki -- Ethical principles in medical research
Ethical foundation – Protect the rights, safety, and well-being of human research participants.
Risk–benefit assessment – Risks must be minimized and clearly outweighed by potential benefits.
Informed consent – Participation must be voluntary, with full, understandable information given.